This is a preview of updates coming to the Technical Bulletin's website in April 2026. Return to current site.
Read more about the modernization release schedule in this announcement.
Comment via the yellow feedback button in the lower right hand corner of the page. Contact the NLM Help Desk with any questions or concerns.
This is archived content.
Links may have become inactive over time. Visit Archive-It to find the original published layout.
Drug Sensor Added to PubMed® Results Page
Dean L. Drug Sensor Added to PubMed® Results Page. NLM Tech Bull. 2008 Jul-Aug; (363):e7.
July 18, 2008 [posted]
August 15, 2008
[Editor's note added]
[Editor's Note: This feature was implemented in PubMed on August 15, 2008.]
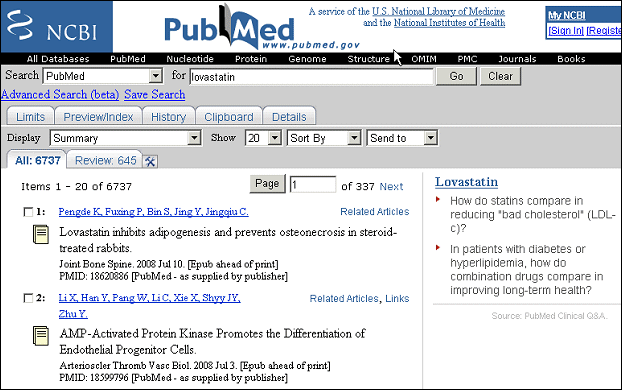
The PubMed Summary results page will soon show results from other high-quality resources in a column to the right of the PubMed search results.
The first example of this new feature will be the Drug Sensor developed at the National Center for Biotechnology Information (NCBI). This sensor detects whether a drug name is present in a user's search, and if so, presents excerpts of information from other resources that you can link to to read more (see Figure 1). The summary consists of a title (created from the drug name in the search query), some content from the linked resource, and an attribution line. At this time, about 200 drug names are included.
The Drug Sensor currently highlights a publication which is new on the PubMed Bookshelf: PubMed Clinical Q&A, a collection of evidence-based medicine summaries. These summaries can be used to learn more about current treatment practices and the level of evidence that supports them.
PubMed Clinical Q&A is based on published systematic reviews. Each PubMed Clinical Q&A summary highlights the key questions identified in the systematic review, gives a brief answer, and links back to the source of the evidence in the results section of the original full-length review.
The first set of summaries is based on systematic reviews from the Drug Effectiveness Review Project (DERP) from Oregon Health & Sciences University. DERP compares classes of drugs, for example, one of their reviews compares the safety and effectiveness of statins. Such collaborations between NCBI and highly respected centers of evidence-based medicine will ensure that PubMed Clinical Q&A will grow into an invaluable resource.